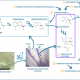
Technical- and risk- and regulatory assessment of NPBTs
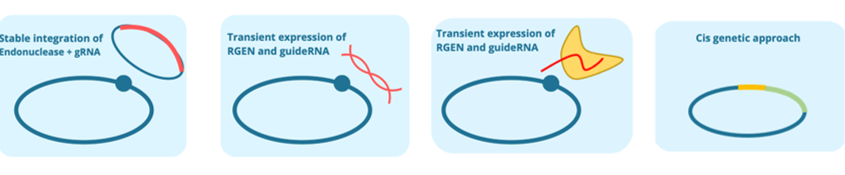
In CHIC project, NPBTs are applied to improve industrial chicory for better inulin and production of health-related terpenes. As part of CHIC, different NPBT approaches to edit chicory genomes were employed. We will technically evaluate these approaches and assess, whether chicory plants generated by the different techniques pose differences on technical level such as off-target rate, efficiency or fall under different regulations. Currently, CHIC partners generate chicory plants with the different approaches. To ensure comparability between the different approaches, all partners work on same target genes.
These approaches are compared in terms of efficiency, time-frame needed and costs. Furthermore, different approaches may show differences in potential off-target activity. We identified potential off-target sites in the genome of the chicory, which could be edited by the used NPBTs. In a first approach, we tested whether identified potential off-targets sites were cleaved in vitro. First results let assume a high specificity of the tested NPBT, as none of the identified off-target sequence was cleaved so far.
Furthermore, it has been screened the current literature systematically together with the ELSA-Gea project (www.dialog-gea.de) and identified thousands of studies wherein NPBTs were successfully applied in a diverse set of more than 40 plant species all over the world. Many studies let assume, that first products are soon touching the commercial market. In many countries, the regulation lags behind this success of NPBTs. Currently, regulation of NPBT differs from country to country. In Member states of the European Union, due to a decision by the European Court of Justice in 2018, plants mutagenized using NPBTs are seen as genetically modified organisms (GMOs). NPBTs are not exempted from the strict European GMO regulation. However, there is an open-ended debate; whether this strict regulation is justified also for NPBTs. Outside Europe other regulations are represented: for several years now, USA, Canada, Argentina, Israel and Chile have a liberal position towards the use of NPBTs. Since CHIC project began in 2018, five more countries clarified the legal position of NPBTs. Among these countries are Brazil, Paraguay, Colombia, Japan and Australia. A number of other countries, e.g. Russia and China, are expected to join soon. Different to Europe, in these countries usually assesses NPBTs derived plants in a case-by-case dependent manner. Mostly they fall out of the scope of regulation, when certain prerequisites were met, e.g. when no foreign DNA was integrated into the edited genome. We are constantly monitoring changes in legislations and follow closely the debate in European Union and its neighbours.

 This project has received funding from the EU Horizon 2020 research & innovation programme under grant agreement N. 760891.
This project has received funding from the EU Horizon 2020 research & innovation programme under grant agreement N. 760891.